The EcoFAB initiative is in close collaboration between Environmental Genomics & Systems Biology (EGSB), Joint Genome Institute (JGI) and Climate & Ecosystem Sciences (EESA) Divisions. EcoFABs, or fabricated ecosystems, are sterile laboratory devices developed in the Northen Lab that enable controlled plant-microbiome studies under conditions where they can be imaged and sampled.
A series of ring trials has been designed to assess the reproducibility of EcoFAB devices by conducting identical experiments across multiple laboratories. In Ring Trial 1, focusing on EcoFAB 1.0, the aim was to validate reproducible outcomes in both plant physiology and soil metabolite depletion. Building on this, the primary goal of Ring Trial 2 is to showcase the consistent formation of the root microbiome, metabolite production, and plant growth across five institutions, utilizing the advanced EcoFAB 2.0 system.
EcoFAB 2.0 ring trial
Participating laboratories: Northen lab and Vogel group at Berkeley lab, Dangl lab at University of North Carolina at Chapel Hill, The Watt Group at University of Melbourne, Schulze-Lefert group at Max Planck Institute, Arsova group at Forschungszentrum Jülich.
EcoFAB 1.0 ring trial
EcoFAB 1.0 ring trial protocol
Sasse, J., Kant, J., Cole, B. J., Klein, A. P., Arsova, B., Schlaepfer, P., … Northen, T. R. (2019). Multilab EcoFAB study shows highly reproducible physiology and depletion of soil metabolites by a model grass. New Phytologist, 222(2), 1149–1160. https://doi.org/10.1111/nph.15662
EcoBOT Team
 |
 |
| Peter Andeer Lawrence Berkeley National Lab |
LT Cornmesser Lawrence Berkeley National Lab |
The EcoBOT consists of a liquid handling robot with a built in, custom growth chamber, imaging stations and a robotic arm that integrates these components. Learn more about the features of the EcoBOT below:
The growth chamber: The EcoBOT growth chamber will have up to three shelves, each with a capacity > 50 EcoFABs with customizable light sources capable of varying day/night cycles, intensity, and wavelengths, and below shelf heating/cooling.
The hyperspectral imaging camera: An imaging system built around a visible range hyperspectral camera (Specim) will collect images in 205 bands from vertical and horizontal positions, enabling image analysis of plants grown in the EcoBOT
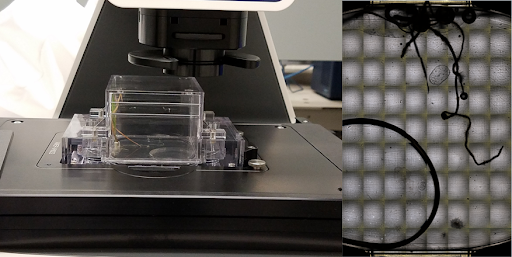
The microscope: The EcoBOT is capable of detailed imaging of the EcoFAB root zones with an EVOS inverted, epifluorescent microscope that can be used to scan roots for morphological changes as well as microbial localization.
The robotic arm: The arm can move an EcoFAB from the growth chamber to any of the listed components, such as the imaging systems or the deck of the robot.
The liquid handling unit: The liquid handling unit can sample and refill EcoFABs aseptically.



Videography: Marilyn Sargent
EcoFAB 2.0 Development and Testing Team
 |
 |
| Peter Andeer Lawrence Berkeley National Lab |
Vlastimil Novak Lawrence Berkeley National Lab |
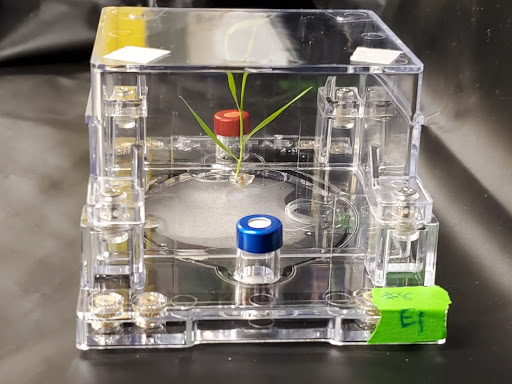
Injection molding allows for mass production and standardization of EcoFAB 2.0 parts for ease of assembly and reproducible experimentation. The chamber enables control of liquid flow through the chamber, spatially defined imaging of the root system, and the ability to sample and add microbes and materials to the root system in a spatially defined manner.
EcoFAB 1.0 Team
 |
| Jian Gao Lawrence Berkeley National Lab |
 The initial design of EcoFAB 1.0 relied on 3D printing of molds used to cast Polydimethylsiloxane (PDMS).
The initial design of EcoFAB 1.0 relied on 3D printing of molds used to cast Polydimethylsiloxane (PDMS).
The EcoFAB initiative was announced in 2015 as a cross-functional team of biologists, geologists, and ecologists from Berkeley Lab to provide critical new insights into ecosystem processes through the creation of controlled model ecosystems in which microorganisms and host responses can be monitored in response to additional or changing variables. Check out the video below to learn more about the EcoFAB.
EcoFAB 1.0 Steering Committee
 |
 |
 |
| Jo Handelsman Wisconsin Institute for Discovery |
Christopher Henry Argonne National Lab |
Kirsten Hofmockel Pacific Northwest National Lab |
 |
 |
 |
| Caroline Masiello Rice University |
Dianne Newman Caltech |
Trent Northen Lawrence Berkeley National Lab |
 |
 |
 |
| James Tiedje Michigan State University |
John Vogel Lawrence Berkeley National Lab |
Karsten Zengler UC San Diego |